Conditioner fabric softener antiseptic soaps. Biodegradable less harmful for envi - harmful bcs of petrochem ingredients.

How Do Detergents And Soaps Work Explain That Stuff
A detergent or surfactant is a chemical compound which has surfactant properties that make it capable to remove soils.

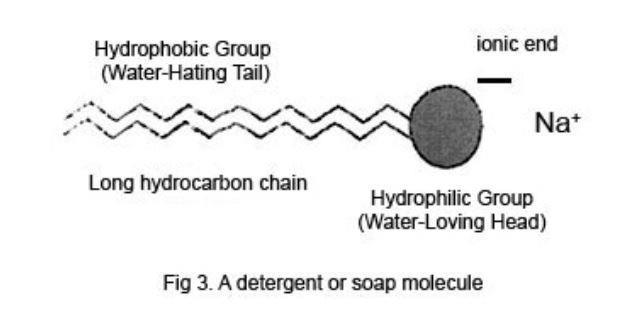
. Bleach may be used separately or may be included in the detergent itself. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. Used natural ingredients - petrochem ingredients.
In the second step soap or detergent is applied to the surface to be absorbed. Cleaning a soiled surface is a four-step process. These wetting agents are best as antiseptics or bacteriostatics.
When selecting a detergent the first consideration is usually the form of the hydrophilic group. The seemingly simple process of cleaning a soiled surface is in fact complex and consists of the following physical-chemical steps. In water a sodium soap dissolves to form soap anions.
A detergent is a sodium salt of long-chain benzene sulphonic acid or sodium salt of long-chain alkyl hydrogen sulfate which has cleansing properties in water. Due to its chemical structure and reactivity a detergent can bind to an oily stain and be washed away in water making it ideal for cleaning. There are a large variety of detergents.
Anionic cationic and non-ionic. The hydrophobic end will bond to oil but not to water. The majority are alky sulfates.
The three types we will examine are. A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. Detergents are surface-active agents surfactants used for industrial and household cleaning and also for other purposes eg as emulsifiers for a variety of products.
The hydrophilic end will bond with water but not to oil. Forms scums caused of water hardness - no scums because BUILDERS. Ionic detergents contain anionic or cationic head groups and possess a.
Most detergents have a negative ionic group and are called anionic detergents. Laundry detergent dishwasher detergent glass cleaner. A hydrocarbon is a molecule that is made of hydrogen and carbon.
Like soaps they contain anionic groups such as sulphonate groups or sulphate groups and long-chain hydrocarbon a non-ionic group. This ability is due to the structure of soaps and detergents. This type of agent wherein the hydrophilic portion is the positive ion is called a cationic agent.
If detached oil droplets and dirt particles did not become suspended in the detergent. Detergent molecules contain a hydrophobic moiety which is soluble in nonpolar materials and a hydrophilic portion which has affinity toward water. Its known to irritate skin eyes and lungs and when it mixes with wastewater it can form toxic organic compounds that have been linked with respiratory issues liver and kidney damage.
Detergents are also known as surfactants as they have the ability to decrease the surface. For cleaning the body - for laundry and other cleaning purposes. The hydrophobic hydrocarbons are repelled by water but are attracted to oil and grease.
The chains love oil and grease and will try to stay away from water. Detergent molecular structures consist of a long hydrocarbon chain and a water soluble ionic group. Arm Hammer Sensitive Skin Free Clear 140 Loads Liquid Laundry Detergent 210 Fl oz.
Soaps and detergents are also called surface-active agents or surfactants. In the first step the surface to be cleaned is made wet with water. AI is known as intelligence expressed by machines.
Also possible are compounds the lipophilic end of which bears a positive charge fig. A detergent molecule has two ends. These compounds are also known as invert or reversed soaps.
Hundred or thousands of yrs - decade ago. A family of soap-like compounds that are more soluble in hard water. The water-fearing end of the surfactant is made up of hydrocarbon chains.
The cleansing action of soaps and detergents. Amphipathic molecules that contain charged hydrophilic or polar groups at the end of long lipophilic hydrocarbon groups are called detergents. A Laundry detergent composition is a formulated mixture of raw materials that can be classified into different types based on their properties and function in the final product.
Often they are the sodium salts of long chain alkyl hydrogen sulphate or a long chain of benzene sulphonic acid. The water-loving end is known as the hydrophilic end. Like soaps detergents have hydrophobic or water-hating molecular chains and hydrophilic or water-loving components.
Start studying the Chapter 11 Quiz flashcards containing study terms like Select the statement that best describes the antimicrobial activity of chlorhexidine Choose the method used to sterilize an inoculation loop used in lab for culturing bacteria The easiest microbial forms to kill or inhibit. Surface active molecules present in soaps and detergents dissolve in water. AI is an extremely complex computer program that can self-learn and make without pre-programming.
The most commonly found detergents are alkylbenzene sulfonates. This is a chemical by-product of detergent manufacturing. The cleansing action of both soaps and detergents results from their ability to lower the surface tension of water to emulsify oil or grease and to hold them in a suspension in water.
Detergents are a class of chemical compounds that are used for cleaning because of their dual hydrophobic and hydrophilic properties. The different classes of raw materials are surfactants builders bleaching agents enzymes and minors which remove dirt stain and soil from surfaces or textiles gave them pleasant feel and odour. Memorize flashcards and build a practice test to quiz yourself before your exam.
Soap and detergent substances that when dissolved in water possess the ability to remove dirt from surfaces such as the human skin textiles and other solids. Based on their structure detergents can be broadly classified as.
Detergents Soaps And Surface Tension Experiment Rsc Education

Solved Question 1 Which Of The Following Picture Might Best Describe A Soap Or Detergent Polar Group 4 Nonpolar Group Mo Ow0 None Of The Above
0 Comments